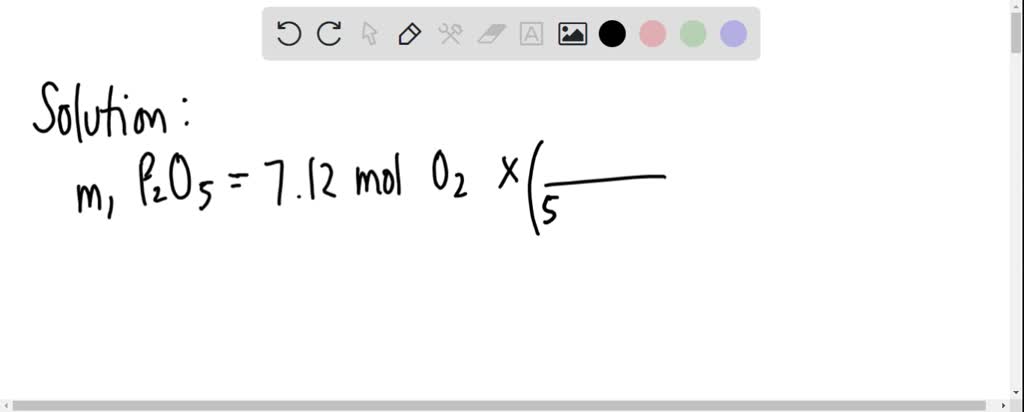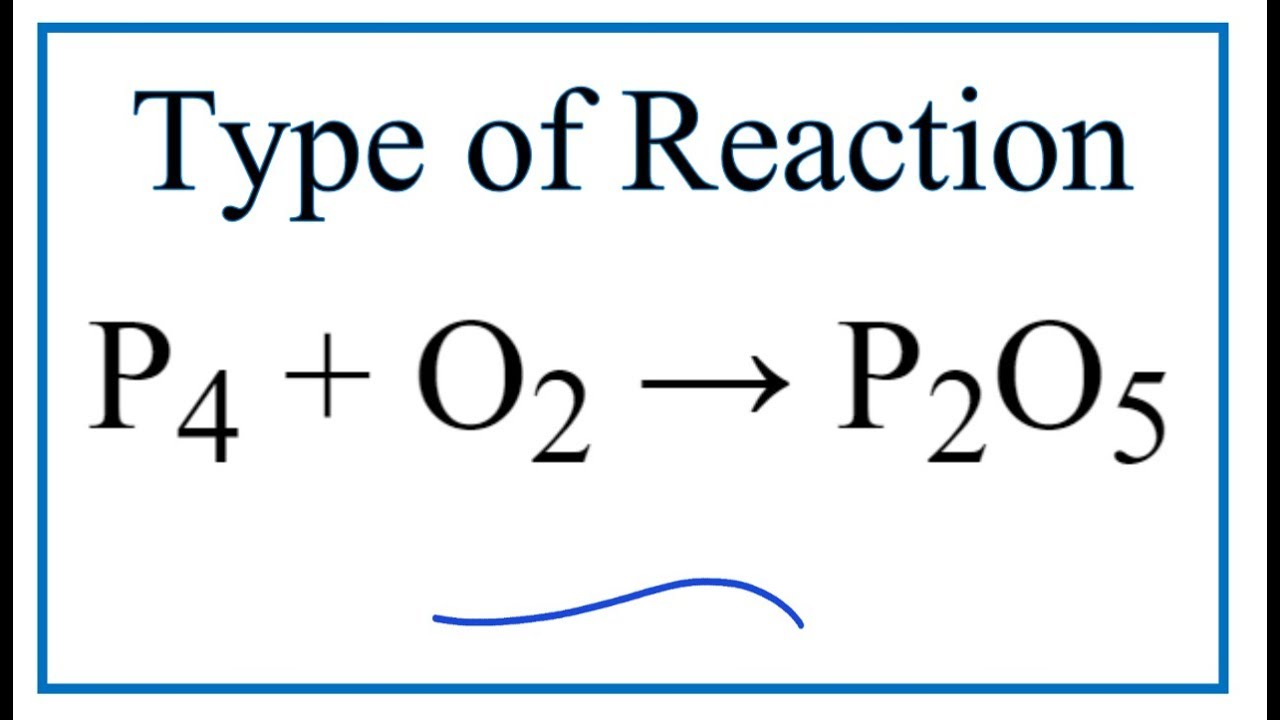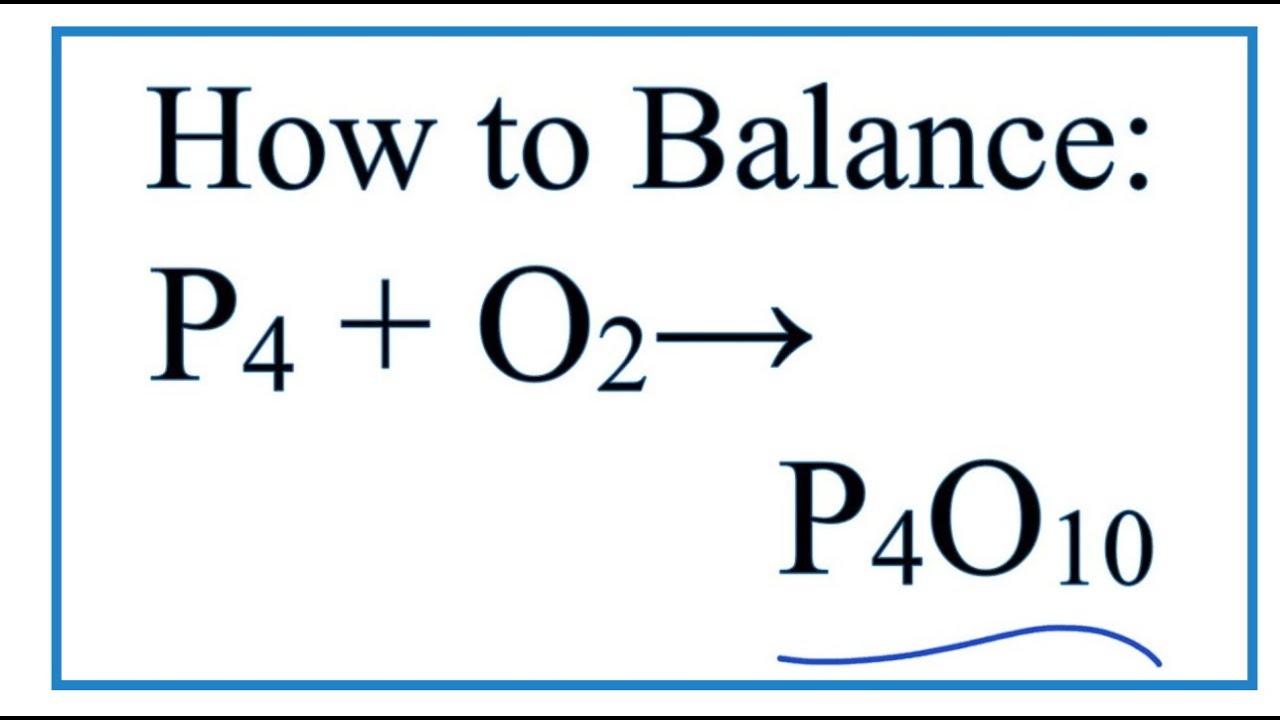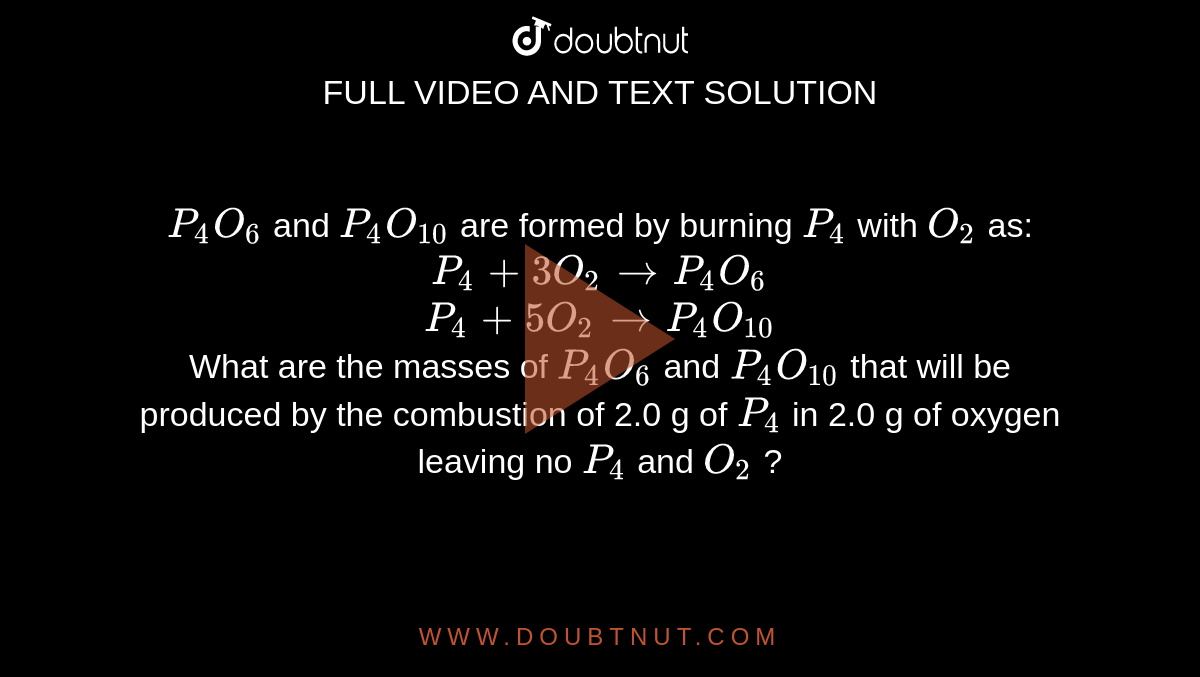
P(4)O(6) and P(4)O(10) are formed by burning P(4) with O(2) as: P(4) + 3O(2) to P(4)O(6) P(4) + 5O(2) to P(4)O(10) What are the masses of P(4)O(6) and P(4)O(10) that will be
For the given reaction P4+5O2 _ p4O10 if 31g of p4 is reacted with excess of oxygen the parentage yield is 60
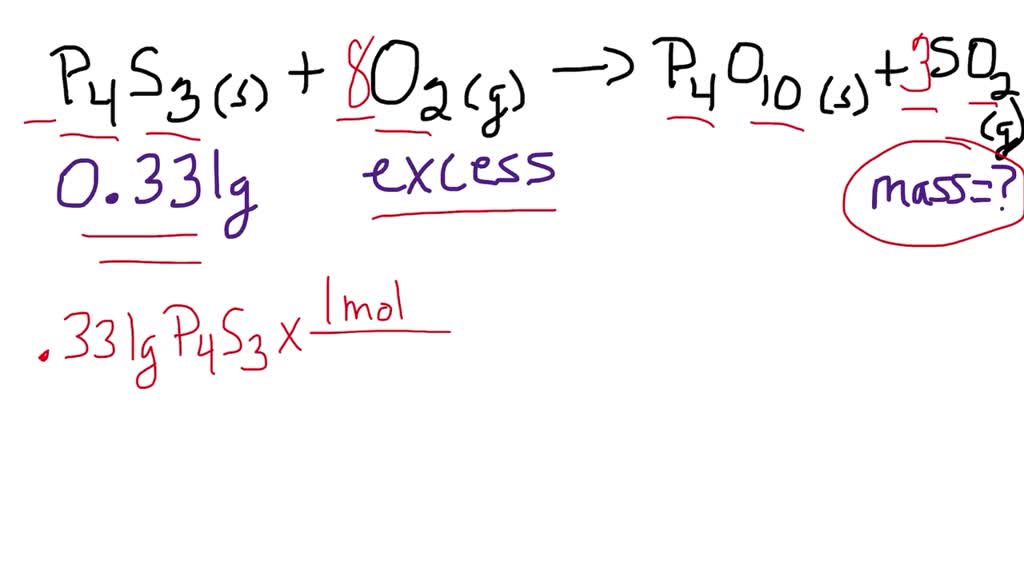
SOLVED: The compound P4 S3 is used in matches. It reacts with oxygen to produce P4O10 and SO2. The unbalanced chemical equation is shown below, P4 S3( s)+O2( g) →P4O10( s)+SO2( g)
a) What is the limiting reactant when 0.200 mol of P4 and 0.200mol of O2 react according to: P4 + 5O2 = P4O10 (b) Calculate the percent yield if 10.0 g of